Benedicts reagent is used to test mainly for the presence of aldehyde and hemiacetal R H CUC citrate 2 - CU 20 s aldehyde Benedicts R Brick - reagent carboxylate red blue solution anion precipitate -7 so option will be correct. A positive test with.
IJ PLS a.
. B I V A - A - HTML Editora IX E. Iodine was used to test for the. A Benedicts solution test b Fehlings solution test c Dye test d Tollens reagent test.
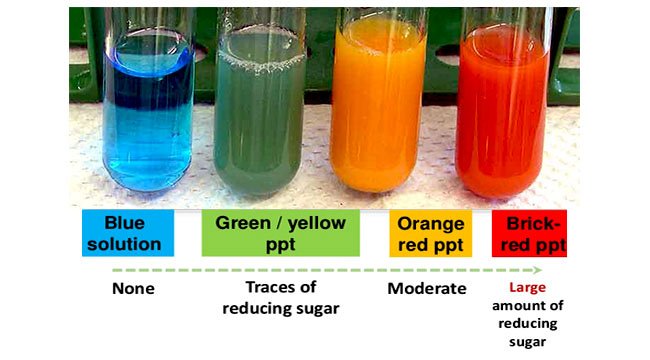
Benedicts reagent can be used to test for the presence of glucose in urine. Significant amount of sugar present the precipitate formed will be an orange-red colour. It is a bright blue solution prepared by mixing copper sulfate pentahydrate CuSO 4.
5H 2 O sodium citrate Na 3 C 6 H 5 O 7 and sodium carbonate Na 2 CO 3 in distilled water 4. The copper ion in the reagent is oxidized as the sugar is reduced. The solution is deep blue in color but the color will eventually change if a substance with reducing sugar is.
The aldehyde group in the sugar is oxidized. Benedicts solution and Fehlings solution are similar in all of the following except. Principle of Benedicts Test.
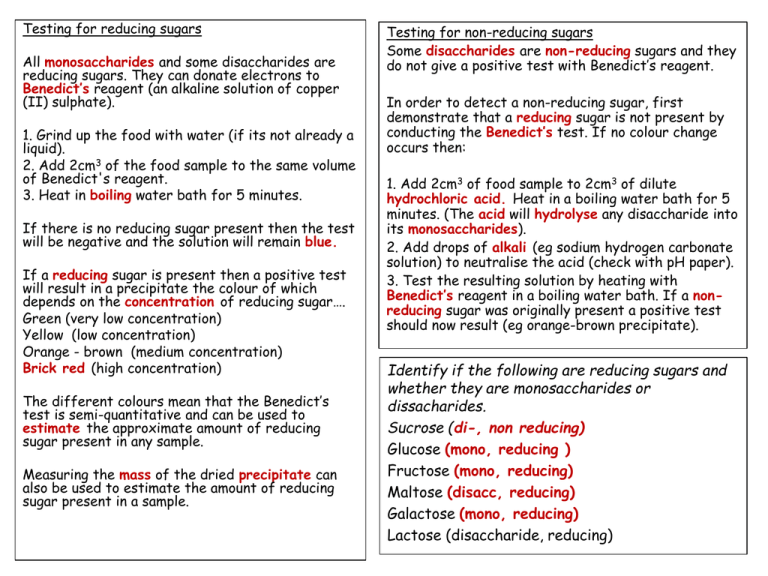
The Benedicts reagent is a mixture of sodium citrate sodium carbonate and copper II sulphate pentahydrate. Will a benedict reagent test be able to detect all sugars. The Benedicts test identifies reducing sugars monosaccharides and some disaccharides which have free ketone or aldehyde functional groups.
Benedicts reagent oxidizes reducing sugars causes a color change. Benedicts reagent is a chemical reagent and complex mixture of sodium carbonate sodium citrate and copper sulfate pentahydrate. The OH groups are exchanged for hydrogens.
Benedicts reagent is used for detecting reducing sugars because _________. It is a bright blue solution that is prepared by mixing copper sulfate pentahydrate sodium carbonate and sodium citrate in distilled water. The principle of Benedicts test is that when reducing sugars are heated in the presence of an alkali they get converted to powerful reducing species known as enediols.
However other reducing substances additionally give a positive response. This test can be used to check for reducing sugars that hold free aldehyde or ketone functional groups. To detect the presence of glucose Benedicts reagent was used.
Question 1 1 pts In Part B of the lab Benedicts reagent was added to each sugar solution. The amino acid which is basic in nature is. Fructose is oxidised by ammoniacal AgNO 3 thus used to identify it.
Benedicts reagent is a compound reagent usually used to identify the presence of reducing sugars. Which one of the following is not used to identify aldehydes. Benedicts reagent is used to identify reducing sugars.
Angelovet April 11 2014. CHCH 3 KOH alcohol Ammonical AgNO 3. Glucose is a reducing sugar and can be detected using Benedicts reagent.
Small amount of sugar present precipitate formed will be a green colour. What color indicated the presence of ketohexoses in your carbohydrate sample. The reducing sugar can be either a monosaccharide or a disaccharide.
Carbohydrates are organic compounds that contain carbon hydrogen and oxygen in a ratio. The copper ion in the reagent is oxidized as the sugar is reduced. Benedicts reagent is a solution of copper sulfate sodium carbonate and sodium citrate in water.
It is often used in place of Fehlings solution to detect the presence of reducing sugars. You test a solution containing an unknown carbohydrate using Benedicts reagent and observe that the solution remains blue. The primary application of Benedicts test is to detect the presence of simple carbohydrates in an unidentified analyte.
The OH groups are exchanged for hydrogens. Benedicts reagent also known as benedicts solution is used in Benedicts test for detecting simple sugars such as glucose. Benedicts reagent is added to the prepared sample containing the glucose and heated to 95C.
The negative control for this reagent remains blue and the positive control turns green yellow or red in that order from least to greatest concentration. A reduction of the. Such tests that use this reagent are called the Benedicts tests.
It is a deep-blue alkaline solution used to test for the presence of the aldehyde functional group. What produces the color that indicated a positive result. Which of the following sugar solutions reacted.
It detects the presence of reducing sugar. Which test would you use to check for its presence in an unknown sample. Which of the following reagent is used to identify fructose.
The aldehyde group in the sugar is oxidized. The reaction of glucose with HI giving n-hexane suggests that all the six carbon atoms are linked in a straight chain as shown in the reaction given below. Benedicts reagent is used to identify.
This incorporates all monosaccharides and numerous disaccharides including lactose and maltose. Among this group of sugars the oxidation of the glucose is the most rapid. Benedicts test is performed by heating the reducing sugar with.
4 Benedict reagent reacts with reducing sugars and due to this reaction there is a. The presence of other reducing substances also gives a positive result. Benedicts reagent test can be used to test for the presence of glucose in urine but this test is not recommended or used for the diagnosis of diabetes mellitus.
Which of the following occurs as a result of this proccess. View the full answer. Glucose in urine is called glucosuria and can be indicative of diabetes mellitus but the test is not recommended or used for the diagnosis of diabetes mellitus.
Each experiment tested for the presence of the following four macromolecules in Parrys vomit. Benedicts Test is used to test for simple carbohydrates. When glucose is heated with Benedicts solution it gives a brick-red precipitate.
Since it gives a positive test with Benedicts solution it must be a monosaccharide but not a lipid. Benedicts reagent is the solution used in Benedicts test to detect simple sugars such as glucose. The reagent used to detect sugar glucose in the urine is benedicts reagent.
What type of carbohydrate is glycogen. Benedicts reagent is used for detecting reducing sugars because _____. For the most part Benedicts test will identify the presence of.

Benedict S Test Principle Preparation Procedure And Result Interpretation

Benedict S Test Reagent Preparation Principle Procedure Reaction

0 Comments